WEDNESDAY, July 8, 2015 (HealthDay News) — Preliminary trial results suggest that an experimental psoriasis drug may control the chronic skin disease better than the current standard treatment.
The drug, guselkumab, was compared to the commonly used medication adalimumab (Humira, Enbrel) in a study involving nearly 300 patients with plaque psoriasis.
Up to 86 percent of patients who received guselkumab cleared their psoriasis or had minimal psoriasis after 16 weeks of treatment, compared to 58 percent of patients taking adalimumab, the researchers reported.
However, patients getting guselkumab were somewhat more prone to infections, the researchers said.
“As a dermatologist, I am particularly excited about the potential of guselkumab and what this investigational therapy may mean for patients and the treatment of moderate to severe plaque psoriasis in the future,” said lead researcher Dr. Kristian Reich, a partner at Dermatologikum in Hamburg, Germany.
The drug works by blocking the protein interleukin-23 (IL23), which plays a role in the immune system and autoimmune diseases such as psoriasis.
The study — the second of three phases of trials needed for drug approval in the United States — shows that blocking IL-23 resulted in significant skin clearance, Reich said.
“These findings provide important insights into the role of IL-23 in psoriasis and the potential therapeutic benefit of guselkumab. My patients specifically like the long injection intervals,” Reich said.
After an initial injection, another one is given at four weeks and again every eight weeks or 12 weeks, he said.
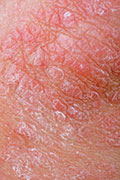
Psoriasis causes itchy, dry and red skin. It also increases a patient’s risk for depression, heart disease and diabetes, among other conditions, the researchers said in background notes with the study. Plaque psoriasis is the most common form of the disease.
Reich said the drug is now being tested in more patients in a phase 3 trial.
“Findings from the ongoing phase 3 trial studies will provide even greater insights into the efficacy [effectiveness] and safety profile of this novel drug,” Reich said.
The trial was funded by the drug’s maker, Janssen Biotech Inc., a subsidiary of Johnson & Johnson. The results were published July 9 in the New England Journal of Medicine.
Dr. Mark Lebwohl, chairman of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, welcomed the trial results. “We have clearly found the critical pathway in the immune system that is responsible for psoriasis,” he said.
For the year-long trial, researchers randomly assigned 293 adults with moderate to severe psoriasis — meaning at least 10 percent of their body was affected — to different doses of guselkumab, adalimumab or a placebo.
They found that after 16 weeks of treatment, patients on guselkumab showed significantly more improvement than those on adalimumab or a placebo.
The improvement in psoriasis among those getting 100 milligrams of guselkumab remained more significant at 40 weeks (77 percent versus 49 percent with adalimumab), the researchers found.
However, over 16 weeks, infections — including appendicitis and lung problems such as pneumonia — were seen in 20 percent of patients taking guselkumab, compared with 12 percent of those taking adalimumab and 14 percent of those taking the placebo, they added.
“Adalimumab is an excellent drug, so it’s particularly promising that the higher doses of guselkumab were even more effective than adalimumab,” Lebwohl said.
This study is important as it highlights the variety of options available for the treatment of psoriasis, said Dr. Katy Burris, a dermatologist at North Shore-LIJ Health System in Manhasset, N.Y.
“It should be kept in mind, though, that this is an early study with somewhat preliminary results, and more work needs to be done before one can fully assess the drug’s safety and efficacy,” she said.
More information
For more on plaque psoriasis, visit the U.S. National Psoriasis Foundation.
Copyright © 2026 HealthDay. All rights reserved.